The COVID-19 pandemic exposed a significant gap in US health security, revealing the need for a rapid detection test for novel and variant pathogens that could potentially be biothreats, while also being able to differentiate between inflammation and infection. Researchers at GW developed a diagnostic test and point of care device called CytoCapture Biomarkers In Situ (CyBIS), which captures human immune cells and measures a specific functional biomarker of infection by either bacteria or virus.
CyBIS uses circulating polymorphonuclear leukocytes (PMNs) as biosensors to detect any pathogenic infection in the body. It can detect biothreats months before a pathogen-specific test could be developed and deployed. CyBIS can rapidly report the number of captured PMNs and whether they are activated. The activation of PMNs is measured by their elastase activity, which is a crucial part of the innate immune response. This enzyme is released from granules and can cleave histones to produce antimicrobial peptides and export chromatin as "Neutrophil Extracellular Traps" (NETs). Unlike traditional diagnostics, which only detect a single pathogen, CyBIS measures the host’s innate immune response to detect infection.
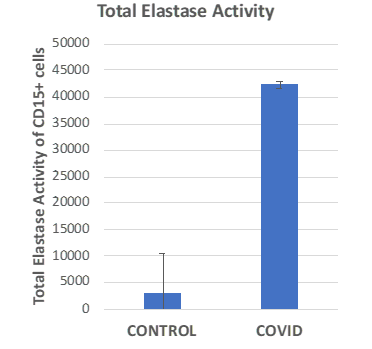
Figure: Comparison of the elastase activity of CD15+ cells (PMNs) in a healthy individual vs. an individual with a COVID-19 infection.
|
Advantages:
- Detection of any type of infection within the body, regardless of the pathogen.
- Results delivered quickly, usually within 30 minutes.
- Increased sensitivity to measure PMN markers, as plasma inhibitors are removed from the sample.
- PMNs can be stored for future analysis.
- Ability to distinguish between infections and other conditions such as sterile inflammation or neurogenic pain disorders.
Applications:
- Point of Care (POC) diagnostic kit/device to detect infection.
- Home-use testing kit/device.
- Protect against bioterrorism by detecting infections by unidentified or uncharacterized pathogens.
- Enable physicians to avoid unnecessary use of antibiotics.