Patients dealing with organ transplants, autoimmune disorders, chronic wounds, ischemic injury, and other diseases that trigger inflammatory processes often lack effective treatment strategies. Macrophages are key cellular components of innate immunity with varying impacts on inflammation. M1 macrophages are pro-inflammatory, while M2 macrophages are associated with anti-inflammatory reactions and tissue remodeling.
GW researchers developed an adoptive cell therapy using immune cells. This therapy is anticipated to suppress local inflammation, enhance wound healing, and enhance tissue regeneration. This groundbreaking technology allows macrophages isolated from mammals to be reprogrammed to the anti-inflammatory M2 phenotype. Macrophages treated with HDAC11 inhibitors result in an increased anti-inflammatory M2 phenotype. Treatment of the cells outside the body rather than treating with HDAC11 inhibitors systemically circumvents any potential adverse effects of HDAC11 inhibitors while fully benefiting from their anti-inflammatory effects via the treated macrophage cells. In vitro proof of concept is complete. Mouse models are underway for in vivo validation.
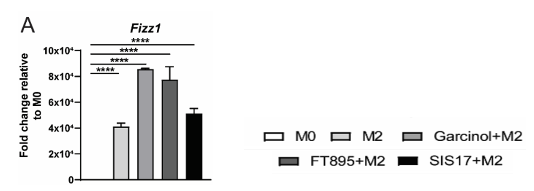
Figure: Pre-treatment with HDAC11 inhibitors (Garcinol, FT895, and SIS17) enhanced the expression of M2 marker Fizz1 showing that HDAC11 inhibition in macrophages strongly favors M2 phenotype.
Applications:
- Cell therapy to enhance wound healing and tissue regeneration,
- Cell therapy to prevent organ transplant rejection, including skin
- Cell therapy to facilitate heart regeneration and repair after ischemic injury.
- Cell therapy to suppress inflammatory and autoimmune disorders
Advantages:
- Uses patient’s own immune cells
- Does not require any genetic engineering of therapeutic cells
- Avoids any potential toxicity of systemic HDAC inhibitor treatment